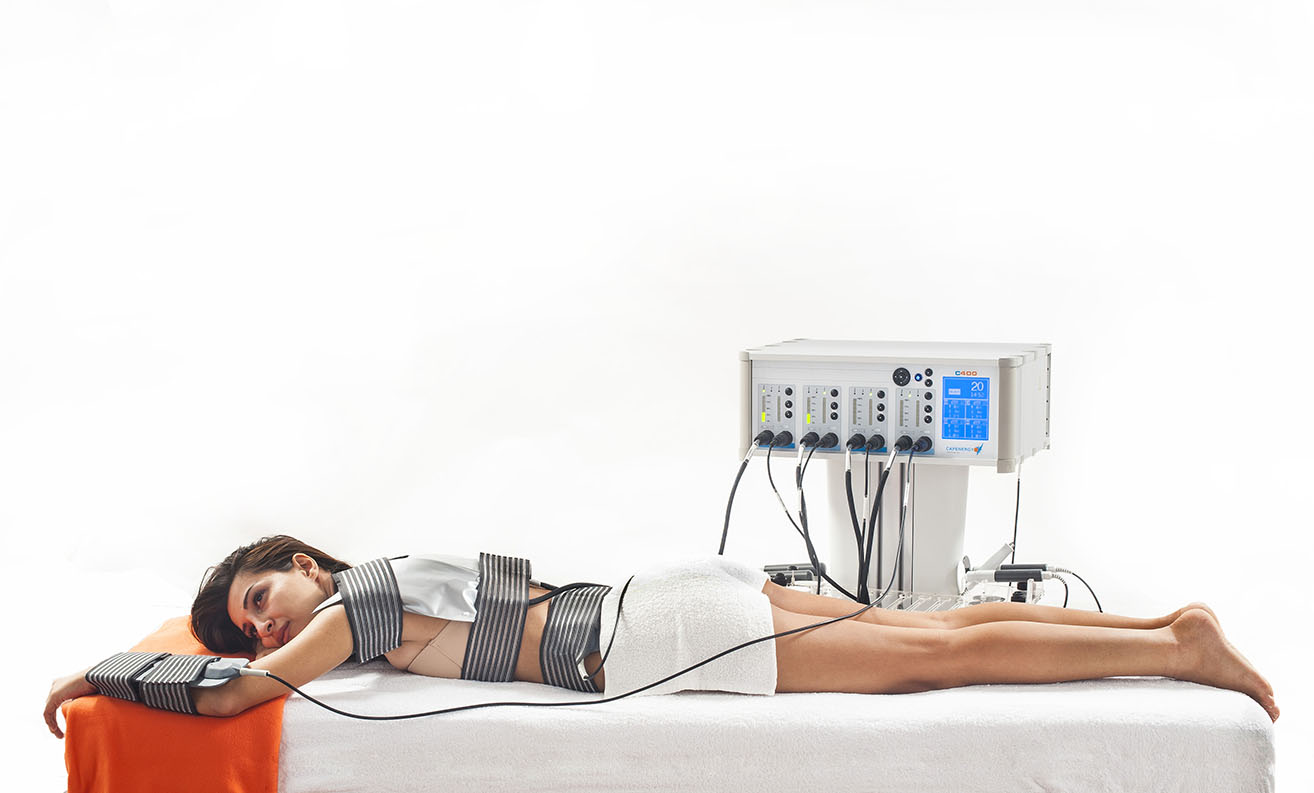
The C200 Respiratory equipment is a high-power, medium-wave, radiofrequency-driven diathermy device with the approved clinical indication for the treatment of asthma, as well as the symptoms derived from it. It is the only device the only tecartherapy device that has this accreditation demonstrated in its Medical CE Certificate.
C200 Respiratory is a technological innovation that enables a rapid response to problems of airway obstruction caused by bronchospasm, inflammation, and the inability to breathe in and out. Thanks to this technology, there is an endothermic increase in body temperature in a localized way, which allows bronchodilation to take place and the pulmonary circulation to increase, which again entails the entry of air and its subsequent exit and an improvement in gas exchange.
The C200 Respiratory device is an innovative non-pharmacological intervention therapy, automated application for treatments of between 15 and 20 minutes, both in infants and adults. It is a therapy with no apparent negative side effects, completely harmless, well tolerated by the patient, easy to apply and automated, non-invasive, in the case of adults, since physical contact between the operator and the patient is not required. Patients who can access this treatment are all those who do not have fever, who do not have pacemakers, and who are not
anticoagulated, in addition to pregnant women. It is an inexpensive, simple and
operational option to improve microcirculation, relax spasm, reduce the feeling of
stiffness in the chest tightness, and thus avoid pulmonary hypoxia. Through a
temperature sensor located in the contact accessories, the device monitors the
progress of the treatment at a thermal level, thereby achieving a more focused
therapeutic target and also guarantees patient safety against the risk of burns.
This innovative technology is a Class IIa medical device, certified by the Notified
Body 1639, and which is manufactured in Spain following an ISO 13485 quality
procedure. .
The C200 Respiratory device can be purchased in its adult modality,
(this being “unattended” or “hands-free” format), for children (being attended by a
qualified professional to adequately adapt the therapy to the child’s anatomy) or
for both (automatic + manual).
CHILD OPTION | PROTOCOL

1. Place the passive plate on the back
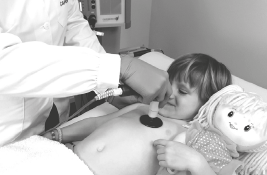
2. Capacitive electrode
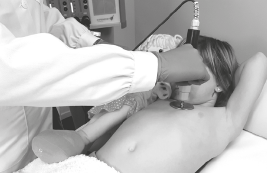
3. Resistive electrode
ADULT OPTION | PROTOCOL

1. Place the passive plates on the back
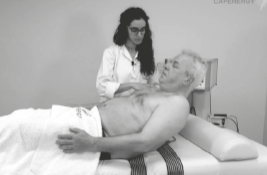
2. Automatic plates ( capacitive & resistive effect )
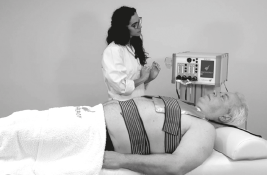
3. Automatic respiratory treatment